
Chemical activity series series#
To the next page in the reactivity series sequence.

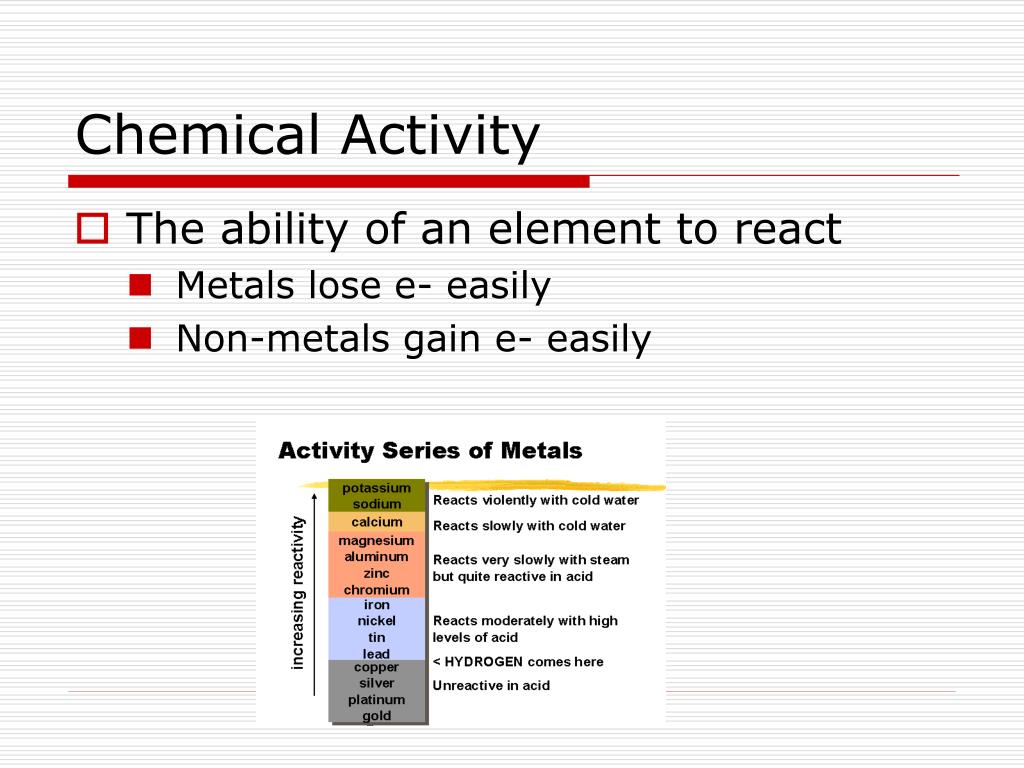
The rest of the Reactivity Series topic looks at how you can build up this list, and its importance in chemistry. Common metals like aluminium and zinc and iron are somewhere in the middle. So sodium and potassium, for example, are extremely reactive, and gold is virtually unreactive. The reactivity series lists the metals (and a couple of non-metals) in order of decreasing reactivity. The table below is an activity series of most common metals, and of the halogens. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. There will be much more detail in further pages in this section. The activity series is a list of elements in decreasing order of reactivity. Will there be a reaction between Br₂ and KCl? Br is below Cl in the series, so we predict no reaction.This page takes a brief look at what the reactivity series consists of. We predict that Cl₂ should replace the I in KI, forming KCl and I₂. We predict that Cu should replace the Ag in AgNO₃, forming Cu(NO₃)₂ and solid Ag.Ĭu(s) + 2AgNO₃(aq) → Cu(NO₃)₂(aq) + 2Ag(s)Ĭl is above I. We predict that Mg should replace the H in HCl, forming MgCl₂ and gaseous H₂.Īlso, Cu is above Ag. This enables chemists to predict which combinations will undergo single displacement reactions.įor example, Mg is above H in the series. (General Science) Metals and Non-Metals: Reactivity Series & Their Chemical Properties. An element that is higher in the series will displace an element that is below it. The hydrogen atoms in HCl are replaced by Zn atoms, and in the.
For example, 2 HCl (aq) + Zn (s) ZnCl2(aq) + H2(g) is an example of a single-replacement reaction.

By referring to a periodic table, we can see that cesium (Cs), potassium (K), and sodium (Na) are in Group 1A and thus more reactive than calcium (Ca) or magnesium (Mg), which are in group 2A. A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products. Thus group 2A metals (alkaline earth metals) are less reactive than Group 1A metals. It lists the metals and nonmetals in decreasing order of chemical activity. The activity of metals decrease as you go right across the period. The general equation for a single-displacement reaction is:Ī and B must be either different metals (including H) or different halogens.Ĭhemists have devised an Activity Series. This is the General Knowledge Question and Answers on Metals its Properties and Reactivity Series which will help in your academic and competitive exams preparations like 10th class, 12th class. In a single-displacement reaction, one element displaces another element in a compound. The activity series is a list of elements in decreasing order of reactivity.


 0 kommentar(er)
0 kommentar(er)
